Quality Management System (QMS)
TrueRCM Quality Management System (QMS)
Product: TrueRCM EHR, Version: v1.0
1. Introduction
TrueRCM maintains a Quality Management System (QMS) to ensure that our health IT solutions meet ONC certification requirements, customer expectations, and regulatory standards. Our QMS is adapted from ISO 9001 principles and tailored to agile software development. It emphasizes continuous improvement, patient safety, and data integrity while maintaining flexibility and responsiveness.
2. QMS Framework
Our QMS governs the entire software development lifecycle (SDLC), from requirements gathering to deployment and post-production support.
Key Elements:
Quality Policy: Deliver secure, reliable, and compliant health IT solutions.
Process Control: Agile ceremonies, code reviews, automated testing, and peer validation.
Risk & Safety Management: Identification and mitigation of safety-related issues (e.g., §170.315(a.1-5, 14); (b.2-3, 11)).
Documentation: Version-controlled repositories for requirements, test evidence, and change logs.
Continuous Feedback: User stories, sprint reviews, and complaint handling feed back into product improvement.
3. QMS Process Flow

Fig. 1: QMS Process Flow
4. Agile Integration with QMS
| Agile Practice | QMS Alignment |
|---|---|
| Sprint planning | Requirements capture & traceability |
| Daily standups | Quality monitoring & progress checks |
| Sprint reviews/demos | Stakeholder feedback → feeds continuous improvement |
| Retrospectives | Root cause analysis & process refinement |
| Automated CI/CD pipelines | Verification, regression testing, compliance checks |
5. Complaint Handling and Corrective Actions
Intake: Complaints logged via ticketing system.
Review: Triaged by QA & compliance team.
Resolution: Fixes prioritized in backlog and verified before release.
Prevention: Root cause analysis during retrospectives; updates to coding standards, test suites, and workflows.
6. Roles and Responsibilities
Product Owner: Ensures regulatory requirements are translated into user stories.
Developers: Follow coding standards, document design decisions.
QA & Compliance Team: Executes safety-enhanced design (SED) tests, maintains evidence.
Management: Reviews metrics, ensures escalation path for safety or compliance risks.
7. Metrics and Continuous Improvement
Defect Density (per sprint)
Test Coverage %
Time to Resolution for Safety-Related Issues
Customer Complaint Resolution SLA
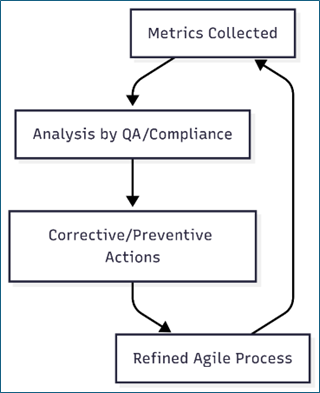
Fig. 2: Metrics and Continuous Improvement
8. Conclusion
Our QMS balances regulatory rigor with agile flexibility, ensuring that our certified health IT solutions are safe, reliable, and continuously improving. The system aligns with ONC certification requirements, supports ISO-aligned quality practices, and provides robust documentation and traceability for audits.